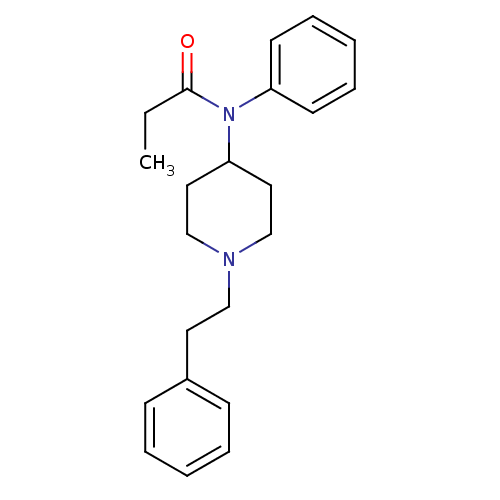
BDBM50008984 4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-phthalazin-1-one::CHEMBL596::Duragesic-100::Duragesic-12::Duragesic-25::Duragesic-50::Duragesic-75::FENTANYL::FENTANYL CITRATE::FENTANYL-HCl::Fentanyl-100::Fentanyl-12::Fentanyl-25::Fentanyl-50::Fentanyl-75::Fentora::Innovar::Ionsys::N-(1-Phenethyl-piperidin-4-yl)-N-phenyl-propionamide::N-(1-Phenethyl-piperidin-4-yl)-N-phenyl-propionamide(Fentanyl)::N-(1-phenethylpiperidin-4-yl)-N-phenylpropionamide::US20230399418, Compound Fentanyl
SMILES CCC(=O)N(C1CCN(CCc2ccccc2)CC1)c1ccccc1
InChI Key InChIKey=PJMPHNIQZUBGLI-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50008984
Found 2 hits for monomerid = 50008984
Affinity DataIC50: 1.48E+5nMAssay Description:Antagonist activity at H1 receptor in human HeLa cells assessed as inhibition of histamine-induced Ca2+ release by using fura-2AM-based fluorescence ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Displacement of [3H]mepyramine from histamine H1 receptor in Sprague-Dawley rat brain membrane after 2 hr by scintillation countingMore data for this Ligand-Target Pair